Abstract
Background Isolated distal deep vein thrombosis (IDDVT) is the most common clinical presentation of DVT. IDDVT in patients with cancer is often associated with a high risk of proximal extensions and recurrent venous thromboembolic (VTE) events. Anticoagulation (AC) can mitigate these risks but is associated with higher risk of bleeding in this patient population. The efficacy and safety of AC for the management of IDDVT in patients with cancer is unclear.
Objectives We sought to assess the rates of recurrent VTE and major bleeding in patients with cancer and IDDVT.
Methods A systematic search of MEDLINE, EMBASE and PubMed as well as conference abstracts of the American Society of Clinical Oncology, American Society of Hematology and International Society on Thrombosis and Haemostasis (inception to June 2, 2022) was performed. Studies assessing adult patients with cancer and IDDVT we included in the analysis.The primary efficacy outcome was recurrent VTE, and the primary safety outcome was major bleeding as defined by the individual studies. Secondary safety outcomes were clinically relevant non-major bleeding (CRNMB) and overall mortality. Incidence rates of the primary and secondary outcomes were pooled using the random effects model and expressed as events per 100 patient-years with associated 95% confidence intervals (CI) using R software (version 4.0.3).
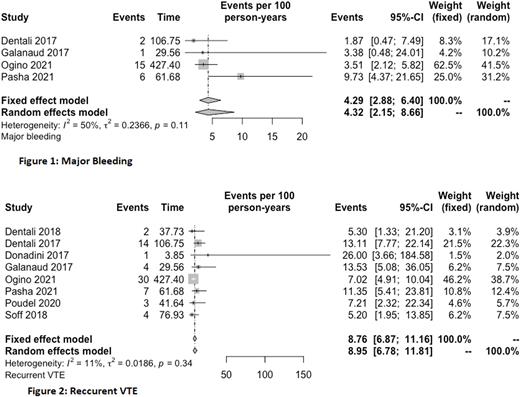
Results After screening 4476 articles, 9 observational studies including 1319 patients (mean age: 66.2±3.7 years, female: 48.6%) with cancer and IDDVT were included in our analysis. The most common cancer types were gastrointestinal tract followed by urogenital and hematological cancers. The incidence rate of recurrent VTE was 8.95 (95% CI: 6.78-11.81) per 100 patient-years (Figure 1). No studies reported the primary outcome based on its location (i.e., recurrent IDDVT, proximal DVT or pulmonary embolism). The incidence rate of major bleeding was 4.32 (95% CI: 2.15-8.66) per 100 patient-years (Figure 2). The incidence rates for CRNMB and mortality per 100 patient-years were 10.68 (95% CI: 5.92-19.29) and 43.09 (95% CI: 33.83-54.89), respectively.
Conclusion Patients with cancer and IDDVT are at high risk of developing recurrent VTE and bleeding complications (both major bleeding and CRNMB). More studies are needed to define the optimal management for this high-risk population.
Disclosures
Wang:Leo Pharma: Research Funding; Servier: Other: Advisory board; Valeo: Other: Advisory board. Carrier:Leo Pharma: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Sanofi: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal